Lithium-ion batteries are popular because they have a number of important advantages over competing technologies:
They're generally much lighter than other types of rechargeable batteries of the same size. The electrodes of a lithium-ion battery are made of lightweight lithium and carbon. Lithium is also a highly reactive element, meaning that a lot of energy can be stored in its atomic bonds. This translates into a very high energy density for lithium-ion batteries. Here is a way to get a perspective on the energy density. A typical lithium-ion battery can store 150 watt-hours of electricity in 1 kilogram of battery. A NiMH (nickel-metal hydride) battery pack can store perhaps 100 watt-hours per kilogram, although 60 to 70 watt-hours might be more typical. A lead-acid battery can store only 25 watt-hours per kilogram. Using lead-acid technology, it takes 6 kilograms to store the same amount of energy that a 1 kilogram lithium-ion battery can handle. That's a huge difference [source: Everything2.com].
That is not to say that lithium-ion batteries are flawless. They have a few disadvantages as well:
They are extremely sensitive to high temperatures. Heat causes lithium-ion battery packs to degrade much faster than they normally would.
Inside a Lithium-ion Battery Pack and Cell:
The lithium-ion cells can be either cylindrical batteries that look almost identical to AA cells, or they can be prismatic, which means they are square or rectangular The computer, which comprises:
One or more temperature sensors to monitor the battery temperature
A voltage converter and regulator circuit to maintain safe levels of voltage and current
A shielded notebook connector that lets power and information flow in and out of the battery pack
A voltage tap, which monitors the energy capacity of individual cells in the battery pack
A battery charge state monitor, which is a small computer that handles the whole charging process to make sure the batteries charge as quickly and fully as possible.
If the battery pack gets too hot during charging or use, the computer will shut down the flow of power to try to cool things down. If you leave your laptop in an extremely hot car and try to use the laptop, this computer may prevent you from powering up until things cool off. If the cells ever become completely discharged, the battery pack will shut down because the cells are ruined. It may also keep track of the number of charge/discharge cycles and send out information so the laptop's battery meter can tell you how much charge is left in the battery.
It's a pretty sophisticated little computer, and it draws power from the batteries. This power draw is one reason why lithium-ion batteries lose 5 percent of their power every month when sitting idle.
Lithium-ion Cells
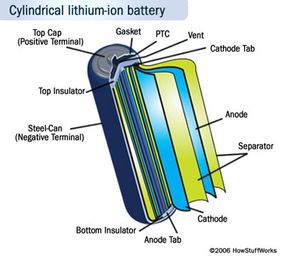
As with most batteries you have an outer case made of metal. The use of metal is particularly important here because the battery is pressurized. This metal case has some kind of pressure-sensitive vent hole. If the battery ever gets so hot that it risks exploding from over-pressure, this vent will release the extra pressure. The battery will probably be useless afterwards, so this is something to avoid. The vent is strictly there as a safety measure. So is the Positive Temperature Coefficient (PTC) switch, a device that is supposed to keep the battery from overheating.
This metal case holds a long spiral comprising three thin sheets pressed together:
-A Positive electrode
-A Negative electrode
-A separator
Inside the case these sheets are submerged in an organic solvent that acts as the electrolyte. Ether is one common solvent.
The separator is a very thin sheet of microperforated plastic. As the name implies, it separates the positive and negative electrodes while allowing ions to pass through.
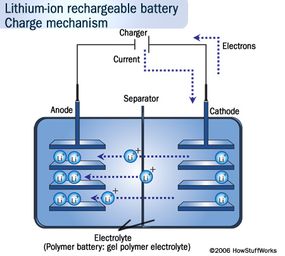
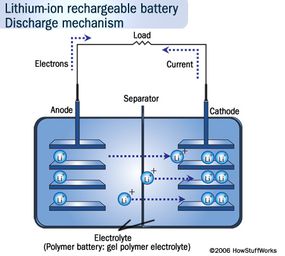
The positive electrode is made of Lithium cobalt oxide, or LiFePO4. The negative electrode is made of carbon. When the battery charges, ions of lithium move through the electrolyte from the positive electrode to the negative electrode and attach to the carbon. During discharge, the lithium ions move back to the LiFePO4 from the carbon.
The movement of these lithium ions happens at a fairly high voltage, so each cell produces 3.7/3.2 volts. This is much higher than the 1.5 volts typical of a normal AA alkaline cell that you buy at the supermarket and helps make lithium-ion batteries more compact in small devices like cell phones. See How Batteries Work for details on different battery chemistries.
 |
 |
scan to wechat:everexceed
