During the long-cycle cycle, the reversible capacity of the lithium-ion battery will continue to decrease due to the reduction of active materials, the precipitation of metal lithium, the continuous consumption of electrolyte, the increase of internal resistance and thermal runaway. Among them, the lithium evolution phenomenon of the graphite negative electrode is the most important cause of battery capacity degradation and internal short circuit.
In the previous article we shared a schematic diagram of the structure of a Li-Cu button battery. A small Pt heating device is placed on the substrate to heat a local area in the battery. The heating device was turned off in the initial stage of the experiment, and the corresponding negative current was attributed to the charging process of the electric double layer on the surface of the Cu sheet and the formation process of SEI. The electric potential is used to overcome the nucleation barrier of lithium metal, and the lithium evolution reaction will not occur. After the heating device is turned on, the output power is 80 mV, and the sudden increase in current can be observed when the temperature is raised to 55°C. Then when the temperature continues to rise up to 95°C, the current further increases to 10 mA. After the experiment, a silver lumps appeared in the central area of the Cu flakes. The SEM and XRD characterization confirmed that the silver material was metallic lithium deposited on the Cu flakes caused by temperature in homogeneity.
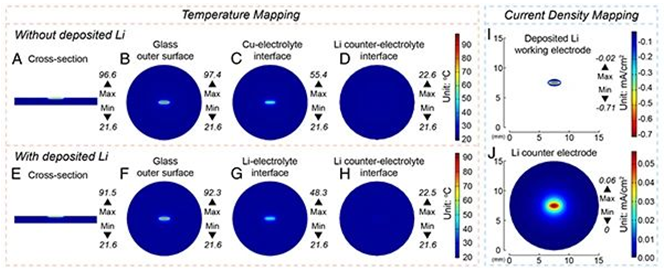
In order to quantitatively analyze the results observed in the experiment, the author uses COMSOL to simulate and analyze the thermodynamics of the battery. Figure 3A-D is the temperature simulation inside the button cell. The highest temperature in the central area of the Cu electrode is 97.4 °C, and it decays rapidly from the radial direction. The temperature of the interface between the Cu foil and the electrolyte is 55.4 °C, and the temperature of the Li foil of the counter electrode is 55.4 °C. Below 22.6 °C, it can be seen from Figure 3E that when lithium deposition occurs, the temperature in the central area drops to 92.3 °C, which is consistent with the experimental observation that the temperature in stage 3 drops from 95 °C to 93 °C. The drop in temperature is due to the good thermal conductivity of lithium metal deposited on the surface, which promotes the dissipation of heat. These results indicate that the deposition of lithium metal can be known in situ by detecting the temperature. From Fig. 3I, an obvious negative current can be observed on the working electrode, which confirms that the reduction reaction of lithium ions to lithium metal has occurred in this region. The high degree of agreement between the experimental results and the simulation proves that the uneven temperature distribution will have a significant impact on the lithium evolution process.
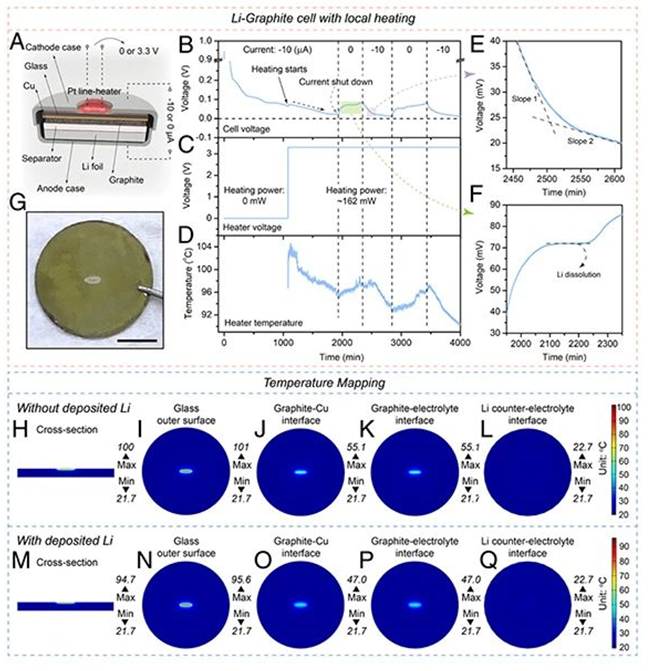
(H-L) Simulation of temperature distribution in various parts of the battery before lithium metal deposition;
Conclusion:
To ensure the smooth operation of your application, EverExceed research and development engineers works day and night to research and design the state of art Lithium Iron phosphate batteries with the perfect charging and discharging parameters which confirms the longest cycle life available for the battery. So choose EverExceed as your brand for the complete reliability.
categories
recent posts
scan to wechat:everexceed
