During the long-cycle cycle, the reversible capacity of the lithium-ion battery will continue to decrease due to the reduction of active materials, the precipitation of metal lithium, the continuous consumption of electrolyte, the increase of internal resistance and thermal runaway. Among them, the lithium evolution phenomenon of the graphite negative electrode is the most important cause of battery capacity degradation and internal short circuit.
By continuation from our last technical article, now we are going to explain more about this phenomenon below.
For the half-reaction of A+ne-→B, the relationship between the temperature coefficient and the equilibrium electrode potential is shown in Equation 1, and the half-reactions of the lithium precipitation process and the graphite lithium insertion process are shown in Equations 2 and 3.
In order to accurately measure the temperature coefficient of the two processes, the author designed a non-isothermal H-type electrolytic cell as shown in Figure 1A. The electrodes on both sides are lithium foil or graphite, and the electrolyte is 1 M LiPF6 EC/DMC, H One end of the type electrode is heated with a temperature-adjustable heating device to form a temperature difference between the two electrodes. Figure 1B and Figure 1C respectively record the change of the open circuit voltage (OCV) of the lithium foil and graphite double electrode over time. As shown in the figure, when ΔV becomes stable, its value is equal to the equilibrium electrode potential under this condition. The temperature coefficient of the equilibrium electrode potential in the lithium analysis process (1.12 mV/K) and the temperature coefficient of the graphite lithium insertion process (0.97 mV/K) are about 0.15 mV/K (Figure 1D). Since the difference in the theoretical equilibrium electrode potential between the lithium-ejection of the electrode and the lithium-intercalation of graphite is about 80 mV, when the internal temperature distribution of the battery is uniform, only when the ambient temperature exceeds 500 ℃, it is possible that the lithium-ejection will occur at the same time during the lithium intercalation process. , This is obviously inconsistent with the actual situation. But if the internal temperature distribution of the battery is not uniform, the situation is quite different. As shown in Figure 1E, the edge area of the electrode is kept at room temperature, and there is no lithium evolution. When the central area is heated by the heating device and the temperature rises by 71 K, the lithium evolution potential will rise by about 80 mV. At this point, from a thermodynamic point of view, lithium ions will be more inclined to extract lithium in the central high-temperature region rather than intercalate lithium in the edge region. Figure 1F further explains the mechanism. The black dashed line is the potential of the graphite anode, the black solid line is the lithium evolution potential, and the gray dashed area indicates that the lithium evolution reaction can proceed spontaneously in thermodynamics. In order to confirm this mechanism, the author further carried out a study on lithium evolution in local high temperature regions on Li-Cu and Li-graphite button batteries.
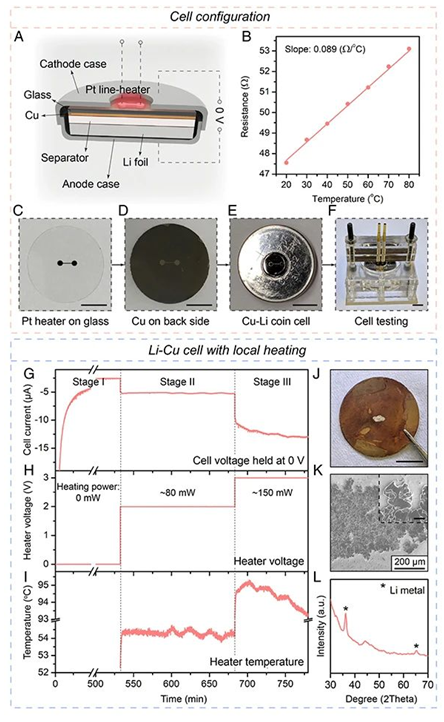
(A) Schematic diagram of Li-Cu button battery with heating device;
categories
recent posts
scan to wechat:everexceed
