With the large-scale popularization of new energy vehicles, lithium-ion batteries are the most important power system. In addition to energy density, cycle stability and safety are also two major problems that need to be improved urgently for commercial lithium-ion batteries. It is generally believed that the phenomenon of lithium evolution is due to the fact that when lithium ions are intercalated on the graphite negative electrode, part of the metallic lithium elementary substance is precipitated on the graphite surface due to kinetic limitations, forming an uneven lithium metal layer. The lithium metal layer on the graphite surface will not only cause serious safety hazards, but also aggravate the growth of the solid electrolyte interface film, making the active lithium trapped in it and becoming dead lithium, unable to participate in the subsequent lithium de intercalation cycle, and the capacity is greatly reduced.
In a series of technical articles we are going to share the details of this phenomenon with you.
Introduction
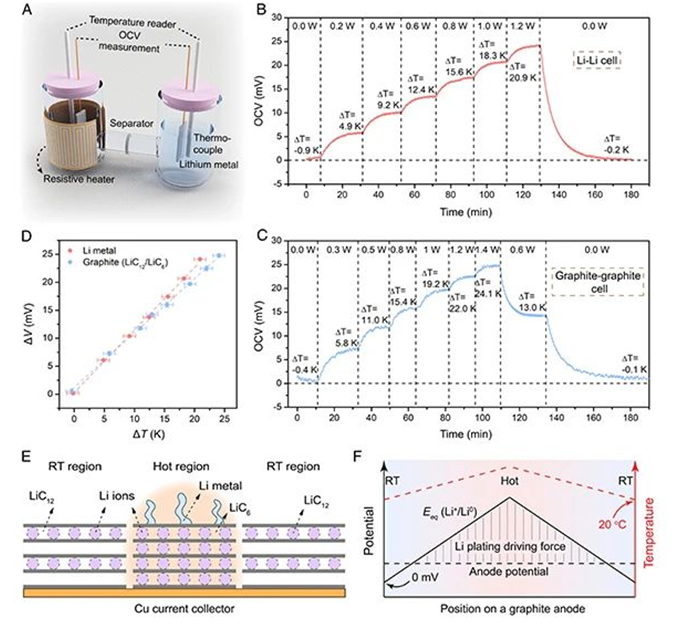
The exothermic reaction and Joule heat accompanying the charging and discharging cycle will cause the internal temperature of the battery to continue to rise, forming a temperature gradient inside the battery, and then changing the equilibrium electrode potential of the redox reaction.
Recently, Cui Yi's group at Stanford University exist PNAS The journal published the title "Under potential lithium plating on graphite anodes caused by temperature heterogeneity “Article. This work confirmed that the temperature in homogeneity inside the lithium ion battery will cause the lithium evolution potential and the lithium insertion potential of the graphite negative electrode part to deviate from the equilibrium electrode potential, and then the precipitation of lithium metal will occur at a potential above 0 V (vs Li0/Li+) . This under-potential lithium evolution phenomenon caused by thermodynamic factors explains well why graphite anodes also exhibit lithium evolution under slow charging conditions. This work is to better understand the uneven lithium deposition behavior and prolong the lithium ion cycle life. Provide a theoretical basis.
Research highlights
1. The thermodynamic factors in the phenomenon of lithium analysis have been ignored by people. This work has studied the abnormal behavior of lithium metal precipitation from the perspective of thermodynamics through in-depth study of the temperature in homogeneity inside the battery.
2. This work proves that as the temperature increases, the precipitation reaction of lithium metal will occur in the local area of the graphite anode at a potential above 0 V (vs Li0/Li+), and the different lithium precipitation modes are analyzed in depth.
3. This work has conducted in-depth research on the under potential deposition of lithium metal on graphite anodes through experiments and simulations, and has a more comprehensive understanding of the phenomenon of lithium evolution. It is hoped that it will guide the improvement of lithium-ion battery fast charging technology.
Graphic guide
categories
recent posts
scan to wechat:everexceed
