When charging
lithium ion battery, Lithium precipitation not only reduces the performance of the battery and greatly shortens the cycle life, but also limits the fast charging capacity of the battery, and may cause disastrous consequences such as combustion and explosion.
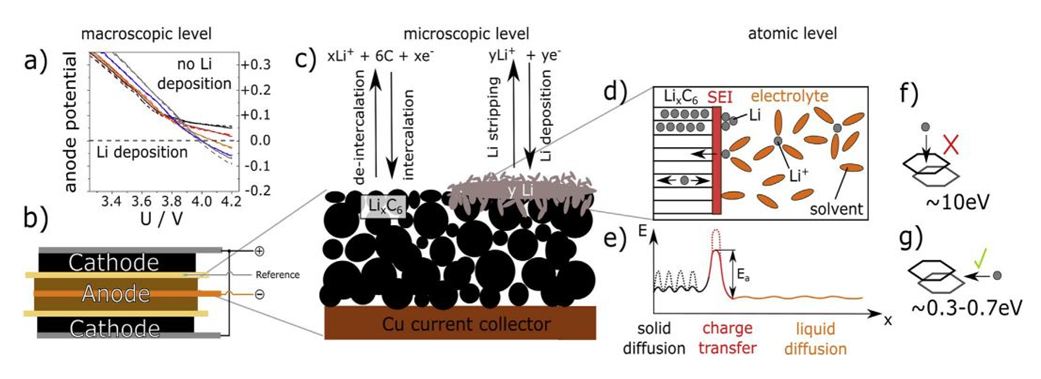
In a series of articles we will discuss about the problems from the macro scale of lithium-ion battery, working conditions, gradient existing in the battery, electrochemical test, safety test, etc.), micro scale (electrode, particle, microstructure, etc.) and atomic scale (atom, ion, molecule, activation energy barrier, etc.). Today we are going to discuss about what factors are affecting the side reactions of lithium deposition:
1.The positive and negative electrodes of the lithium-ion battery and the metal lithium reference electrode form a three electrode system as shown in Fig. 1 for charging test. It is found that the higher the state of charge (SOC) and charging current density, the lower the test temperature, the more negative the potential of graphite negative electrode, and the more prone the side reaction of lithium deposition on the negative electrode surface.
2.Lithium battery level: increasing the N / P ratio within a certain range helps to limit the charge state of the negative electrode to a lower level, so as to reduce the battery aging rate and make the internal resistance of the battery increase more slowly.
3.Negative electrode reaction kinetics: lithium evolution reaction is also affected by the type, morphology and conductivity of negative electrode materials. They affect the degree of negative polarization from the perspective of diffusion mass transfer or charge transfer, thus affecting the negative potential and negative reaction.
4.Activation energy: the activation energy that solvated lithium ions need to overcome in the diffusion of electrolyte can be ignored, while the activation energy that solvated lithium ions need to overcome in the process of desolvation, diffusion through SEI membrane and charge transfer is the highest. With the progress of the charging process, the number of Li + embedded in the negative electrode gradually increases, the activation energy to be overcome when Li + diffuses in the negative active material increases, and the solid-phase diffusion is more difficult.
5.Temperature: according to Arrhenius formula, when the battery circulates at low temperature, the lithium evolution reaction has a greater reaction rate than the lithium intercalation process, that is, the negative electrode is more prone to lithium evolution reaction at low temperature. This has been verified by the experimental observation that the negative potential of graphite is more negative at low temperature. In addition, the charge transfer and solid-phase diffusion are slower at low temperature, and the reaction rate between metal lithium deposited on the negative electrode surface and electrolyte will also decrease.
6.Charging rate: the charging current rate determines the lithium ion flux on the negative electrode material per unit area. When the solid-phase diffusion process of Li + in the negative electrode is slow (for example, when the temperature is too low, the state of charge is high, or the diffusion of Li + in the material needs to overcome the large activation energy), and the charging current density is too high, the lithium evolution reaction will occur on the surface of the negative electrode. When other conditions remain unchanged and the current density increases to a certain threshold, the negative potential will become negative, accompanied by the beginning of lithium evolution reaction.
7.Others: whether lithium evolution reaction occurs on the negative electrode surface is determined by three factors: charging rate, temperature and state of charge. For example: (1) charging at low temperature does not mean that lithium evolution reaction will occur on the negative electrode. The lithium evolution reaction occurs only when the state of charge and / or current density exceed a certain threshold. (2) In the charging process of lithium-ion battery, if a higher charging current density is adopted when the state of charge is low and a lower charging current density is adopted when the state of charge is high, the lithium evolution reaction can be effectively inhibited.
Conclusion:
To ensure the smooth operation of your application, E
verExceed research and development engineers works day and night to research and design the state of art
Lithium Iron phosphate batteries with the perfect charging and discharging parameters. So choose EverExceed as your brand for the complete reliability.

