Resistance is a physical quantity that characterizes the degree of obstruction of circuit elements to current transmission. The internal resistance (internal resistance) of
lithium batteries is one of the important indicators to evaluate the performance of batteries. In practical applications, the internal resistance of lithium batteries has three important roles:
1. It can be used to evaluate the health of the
battery and predict the battery life.
2. It can be used to estimate the SOC of the battery.
3. The connection status of the circuit in the battery module can also be identified by measuring the internal resistance, and timely judgment can be made when the connection is loose.
When the current passes through the electrode, the phenomenon that the electrode deviates from the equilibrium electrode potential is called the polarization of the battery, and the polarization generates the overpotential. Understanding polarization is important for understanding the internal resistance of the battery, and they are corresponding relationships. In lithium batteries, polarization can be divided into three categories according to the cause of polarization:
1. Ohmic polarization: the battery is composed of electrode materials, electrolyte, diaphragm and various parts, ohmic polarization is caused by the resistance of the battery connected to each part, the voltage drop value follows the Ohmic law, the current is reduced, the polarization is immediately reduced, the current stops immediately disappear.
2. Electrochemical polarization: After the battery is powered on, the electrode surface produces an electrochemical reaction, at this time, the charge transfer rate of a step in the electrochemical reaction process does not reach the impedance of the external discharge rate, the battery must allocate a certain voltage to meet the activation energy of its transfer rate. As the current decreases, polarization decreases significantly in microseconds. Correspondingly, electrochemical polarization produces electrochemical internal resistance, also known as charge transfer impedance.
3. Concentration polarization: Due to the consumption of reactants caused by the electrode surface can not be supplemented in time, resulting in ion concentration difference on the reaction surface, which is the result of material transfer, that is, concentration polarization. This polarization decreases with the current, decreasing or disappearing on the macro second scale (a few seconds to tens of seconds). Correspondingly, concentration polarization produces concentration internal resistance, also known as lithium ion migration impedance.
On the time scale, the ohmic polarization is completed instantaneously, the electrochemical polarization is completed at the microsecond level, and the concentration polarization is completed at the second level.
Several related concepts:
1. Ohmic internal resistance: Ohmic polarization produces ohmic internal resistance.
2. Internal resistance of polarization: the resistance caused by polarization during electrochemical reaction, including the resistance caused by electrochemical polarization and concentration polarization, and the polarization capacitor in parallel to form a resistance loop, used to simulate the dynamic characteristics of the battery polarization generation and elimination process.
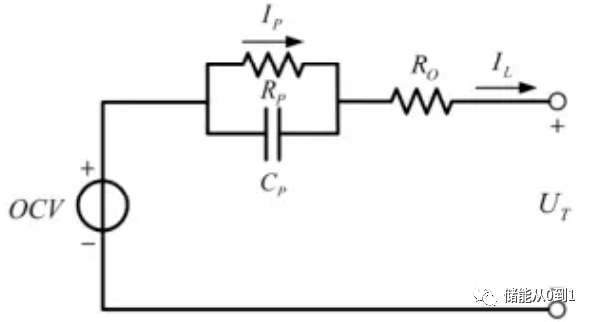
Batteries can be approximated by the Thevenin equivalent circuit model, also known as the first-order model, and their connection relationships can be shown in the figure below. Where, OCV is the open circuit voltage of the battery, Ro is called the ohm internal resistance, Rp is the equivalent polarization internal resistance, Cp is the equivalent polarization capacitance.
Generally, the test results commonly used by enterprises are divided into two categories: 1. Communication internal resistance; 2 DC internal resistance
Ac internal resistance: The AC internal resistance is to inject sinusoidal current signal I=Imaxsin(2πft) into the positive and negative electrodes of the battery, and at the same time, by detecting the voltage drop U=Umaxsin(2πft+ψ) at both ends of the battery, the AC impedance of the battery can be derived; Generally, the sinusoidal AC current signal of 1kHz is input to the positive and negative terminals of the battery, and the parallel value of the Rp and Cp of the battery at this frequency is generally small (note: because the capacitor is approximately short-circuited under the high-frequency signal), which can be ignored. Therefore, the resistance detected by the AC current signal is relatively close to the value of the ohm internal resistance Ro, and the AC internal resistance can generally be considered as the ohm internal resistance of the battery; In the battery production line, the internal resistance meter is often used to measure the internal resistance of the battery, and the AC resistance is measured, which is mainly used to evaluate the production process of the battery core. Through the voltage waveform, the coating effect of positive and negative electrode materials can be evaluated, and the electrode welding effect can be improved.
Dc internal resistance: DC internal resistance is to apply a DC signal to the battery to test the battery internal resistance, generally a constant current pulse current. The DC internal resistance can generally be considered as the ohm internal resistance + charge transfer impedance + lithium ion migration impedance of the battery (the difference in test methods will lead to the absence of concentration polarization, so it may only contain the ohm internal resistance + charge transfer impedance).
The ohm internal resistance is related to the size, structure and assembly of the battery, and its resistance value has nothing to do with the charge and discharge state, and is almost unaffected by the SOC state.
The polarization internal resistance occurs only during the charge and discharge process of the battery, and the polarization internal resistance is affected by the state of SOC. When the SOC of the battery is close to 0% or 100%, its internal resistance to polarization is large, and when the SOC is between 20% and 80%, its internal resistance to polarization is relatively small. And this phenomenon will gradually increase with the increase in the number of battery cycles. Because after many cycles of the battery, the interface between the electrode active substance and the electrolyte of the lithium-ion battery gradually degrades, resulting in an increase in electrochemical impedance.
Test method of DC internal resistance:
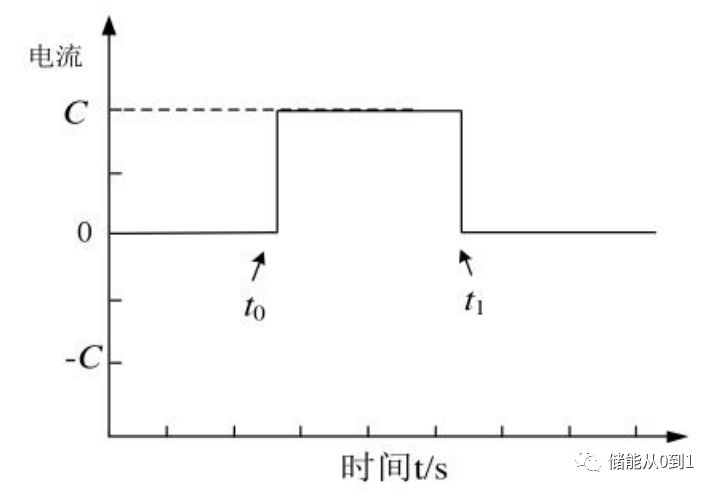
After the end of the discharge process, the voltage of the battery will rebound due to the existence of polarization. The DC impedance measurement is to calculate the internal resistance of the battery by using the voltage difference between the voltage at the moment before the end of discharge and the voltage after the end of discharge. Specifically, the battery is discharged with a constant current of size I, as shown below:
Record and draw the curve of battery terminal voltage over time, and collect the voltage drop and voltage rise of the battery, as shown in the figure below: At time t0, the initial stage of discharge is entered. Due to the existence of ohm internal resistance, the battery terminal voltage drops from point A to point B, and then enters the discharge stabilization stage until the voltage drops to point C (time t1). At this time, due to the interruption of the current, the ohm internal resistance voltage drop disappears, and the voltage can be observed to rise to point D. At the same time, due to the existence of the polarized capacitor, the capacitor voltage cannot change, and the battery voltage gradually recovers and enters the discharge recovery stage, until the polarized capacitor at point E is discharged, and the battery terminal voltage does not change.
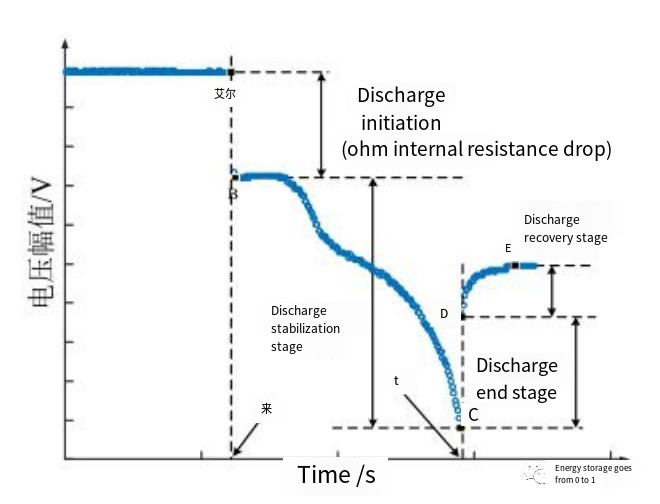
The DC internal resistance is equal to C-> Phase E battery terminal voltage change divided by the discharge current I.
Test method of polarization resistance:
Refer to the figure above, in the discharge recovery stage, the voltage at both ends of the polarization capacitor Cp does not change sharply, and is equal to the polarization resistance Rp voltage, its value is the value of the battery voltage recovery stage, and the current flowing through the polarization resistance Rp before stopping discharge is the discharge current I. Therefore, the polarization resistance Rp can be passed by D-. The calculation formula of terminal voltage change in phase E is as follows: terminal voltage change divided by discharge current I.