The process is the same for all types of
lead-acid batteries: flooded, gel and AGM. The actions that take place during discharge are the reverse of those that occur during charge.
The discharged material on both plates is lead sulfate (PbSO4). When a charging voltage is applied, charge flow occurs. Electrons move in the metal parts; ions and water molecules move in the electrolyte.
Chemical reactions occur at both the positive and negative plates converting the discharged material into charged material. The material on the positive plates is converted to lead dioxide (PbO2); the material on the negative plates is converted to lead (Pb).
Sulfuric acid is produced at both plates and water is consumed at the positive plate.
If the voltage is too high, other reactions will also occur. Oxygen is ripped from water molecules at the positive plates and released as a gas. Hydrogen gas is released at the negative plates—unless, oxygen gas can reach the negative plates first and “recombine” into H2O. A battery will “gas” near the end of charge because the charge rate is too high for the battery to accept. A temperature-compensating, voltage-regulating charger, which automatically reduces the charge rate as the battery approaches the fully charged state, eliminates most of this gassing. It is extremely important not to charge batteries for long periods of time at rates which cause them to gas because they use water, which in sealed valve regulated batteries cannot be replaced. Of course, no battery should be overcharged for a long period of time…even at low rates using so-called “trickle charges.”
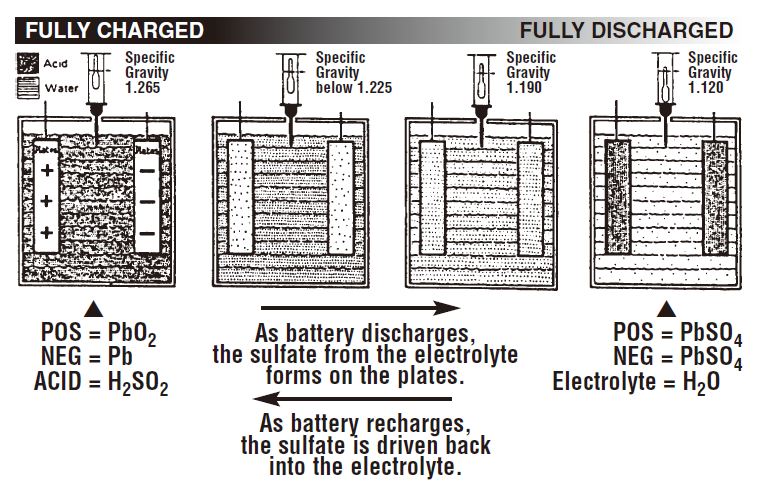
In a fully charged battery, most of the sulfate is in the sulfuric acid. As the battery discharges, some of the sulfate begins to form on the plates as lead sulfate (PbSO4). As this happens, the acid becomes more dilute, and its specific gravity drops as water replaces more of the sulfuric acid. A fully discharged battery has more sulfates in the plates than in the electrolyte.
The following illustration shows the relationship between specific gravity readings and the combination of the sulfate from the acid with the positive and negative plates at various states of charge.
How critical is recharge voltage? Why are all VRLA batteries so charge sensitive?
All lead-acid batteries give off hydrogen from the negative plate and oxygen from the positive plate during charging.
VRLA batteries have pressure-sensitive valves. Without the ability to retain pressure within the cells, hydrogen and oxygen would be lost to the atmosphere, eventually drying out the electrolyte and separators.
Voltage is electrical pressure. Charge (ampere-hours) is a quantity of electricity. Current (amperes) is electrical flow (charging speed). A battery can only store a certain quantity of electricity. The closer it gets to being fully charged, the slower it must be charged.
Temperature also affects charging. If the right pressure (voltage) is used for the temperature, a battery will accept charge at its ideal rate. If too much pressure is used, charge will be forced through the battery faster than it can be stored. Reactions other than the charging reaction occur to transport this current through the battery—mainly gassing.
Hydrogen and oxygen are given off faster than the recombination reaction. This raises the pressure until the pressure relief valve opens. The gas lost cannot be replaced. Any VRLA battery will dry out and fail prematurely if it experiences excessive overcharge.
Note: It is the pressure (voltage) that initiates this problem— a battery can be “over-charged” (damaged by too much voltage) even though it is not fully “charged.”
This is why charging voltage must be carefully regulated and temperature compensated to the proper values.

